Introduction
Early identification of patients (pts) with relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL) at high risk for treatment failure may allow for interventions to improve outcomes; however, known prognostic factors are inadequate. Circulating tumor DNA (ctDNA) has demonstrated the ability to identify previously untreated DLBCL pts at high risk of relapse (Kurtz et al. 2018). We assessed the potential for ctDNA to identify pts with R/R DLBCL receiving bendamustine and rituximab (BR) +/- polatuzumab (pola) at higher risk for disease progression.
Methods
GO29365 (NCT02257567) is a Phase Ib/II study comparing pola+BR versus BR in pts with transplant-ineligible R/R DLBCL and enrolled 80 pts (n=40 per randomized arm). The primary efficacy objective was independent review committee (IRC)-assessed complete response (CR) rate at primary response assessment (PRA, 6-8 weeks after Cycle 6, Day 1 or last dose of study drug). ctDNA was measured in available cohort samples using a customized DLBCL panel on a modified ctDNA CAPP-Seq workflow developed based on the prototype assay reported in Kurtz et al. 2018, with improvement of sensitivity and specificity. ctDNA was reported as mean mutant molecules per mL (MMPM). Plasma depleted whole blood from baseline was used as a source of germline DNA to filter non-tumor-specific variants. A total of 43 samples were available at baseline; eight pts without a paired germline were excluded from efficacy and correlative analyses. Of the 35 samples (n=21 Pola+BR; n=14 BR) with available germline data, paired samples were available for 25 pts at PRA.
Detectable ctDNA (+/-) was determined using empirical p-values (<0.05) through a bootstrap algorithm (Newman et al. 2014, Pati et al. 2018). The proportions of pts with detectable ctDNA are reported by visit with 95% Clopper-Pearson binomial confidence intervals (CI). A Kruskal-Wallis test was used to compare ctDNA levels by response. Univariate and multivariate cox regression was used to correlate ctDNA levels with progression-free survival (PFS) and overall survival (OS). Results are reported descriptively without multiple testing correction.
Results
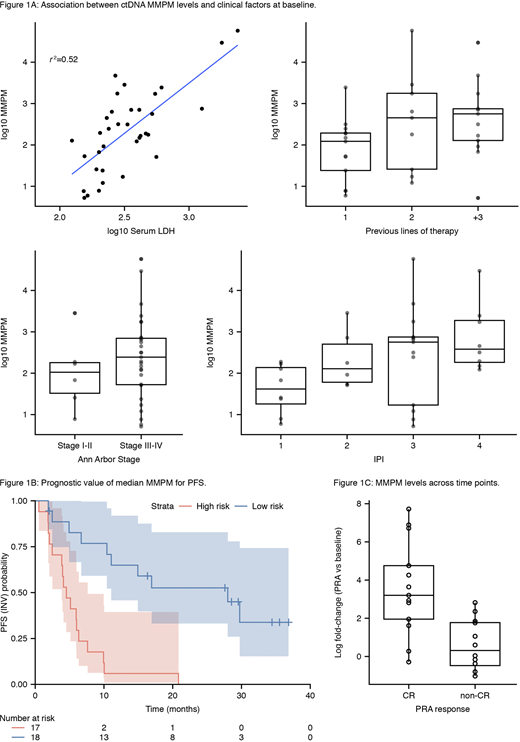
Intent-to-treat and biomarker evaluable populations were similar in demographics and efficacy, but there was a higher proportion of pola+BR versus BR pts assayed (60% vs 40%). Analyses are reported for the pooled arms, but results were consistent across both arms. ctDNA was detected in all available baseline (n=43) samples (95% CI: 92-100). Baseline ctDNA levels were correlated with known prognostic factors including International Prognostic Index (IPI), lactate dehydrogenase (LDH), Ann Arbor stage and number of prior therapies (Figure 1A).
Higher ctDNA levels at baseline were negatively prognostic; when stratifying pts by median ctDNA levels the hazard ratio (HR) for PFS was 0.16 (95% CI: 0.07-0.41; Figure 1B); OS HR 0.23 (95% CI: 0.10-0.52). Similar results were observed for other quartile stratifications. This significant trend was maintained when adjusted in multivariate analysis for treatment, IPI >3, and LDH > upper limit of normal with an adjusted HR for PFS of 0.24 (95% CI: 0.07-0.81) and for OS an adjusted HR of 0.30 (95% CI: 0.09-0.97).
Similar to the first-line setting (Kurtz et al. 2018), on-treatment log fold-changes in ctDNA trended with PRA response (Figure 1C); pts with a CR had a significantly greater average decrease in MMPM across timepoints than non-CR pts (Wilcoxon p-value <0.001). At PRA, four pts (three in pola+BR, one in BR) had cleared ctDNA, potentially due to treatment, all of which achieved CR. ctDNA was detectable in the remaining patients (n=25, 84%, 95% CI: 64-95). There was no clear trend between log fold reduction of ctDNA at PRA and PFS in this cohort, though this analysis is limited by sample size.
Conclusions
Baseline ctDNA levels were correlated with standard clinical risk factors, and were shown to have independent prognostic value for response, PFS and OS in R/R DLBCL pts treated with BR +/- pola. The degree of ctDNA change upon treatment was also correlated with response, but association with PFS needs further investigation with a larger sample size. This provides early evidence that ctDNA can be used to improve identification of R/R DLBCL pts at high risk for disease progression/relapse.
Herrera:AstraZeneca: Research Funding; Karyopharm: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Immune Design: Research Funding; Seattle Genetics: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Pharmacyclics: Research Funding; Merck: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Other: Travel, Accomodations, Expenses, Research Funding. Tracy:F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Croft:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Opat:Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZenca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyme: Research Funding; F. Hoffman-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel accomodations, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ray:F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Musick:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Roche/Genentech, Inc.: Current Employment. Paulson:F. Hoffmann-La Roche Ltd.: Current Employment, Current equity holder in publicly-traded company. Sehn:Chugai: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Teva: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Genentech, Inc.: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Verastem Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy, Honoraria. Jiang:F. Hoffmann-La Roche: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal